Electrocatalysis and Bioelectrocatalysis LAB (EBLab)

GOAL of EBLab:
- research and development of electrocatalysts for several electrochemical reactions based on platinum group metal-free materials pursuing biomimicking and bionspired approaches within the core of the circular economy;
- research and development of bacterial and enzymatic bioelectrochemical systems from fundamental to application.
Platinum Group Metal-free electrocatalysis
Noble-containing and/or critical raw materials-containing electrocatalysts are used in diverse electrochemical applications for fuel production and energy conversion named electrolyzers and fuel cells. The high cost and scarcity of these materials on the Earth crust hinder or severely limit their deployment for large scale commercialization of these electrochemical devices which are generally the base of the green energy revolution. Alternatives need to be pursued. We are replacing the costly platinum group metal (PGM) electrocatalysts with more affordable Earth abundant transition metals (PGM-free) achieving similar electrocatalytic activity using biomimicking and bionspired synthesis methods and approaches and when possible also valorizing waste (e.g. waste biomass and plastic) as precursors during synthetic processes within the core of the circular economy. We are currently synthesizing PGM-free electrocatalysts for reaction such as Oxygen Reduction Reaction (ORR), Hydrogen Evolution Reaction (HER), Hydrogen Oxidation Reaction (HOR), Alcohol Oxidation Reaction (AOR), Oxygen Evolution Reaction (OER). Other reactions of interest within the decarbonization are also CO2 electroreduction (CO2ER) and Nitrogen Reduction Reaction (NRR).
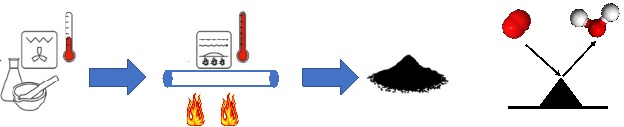
Bio-Electrocatalysis
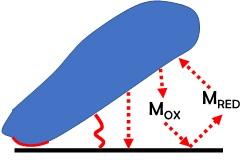
Microbial Electrochemical and Enzymatic Electrochemical technologies have captured the attention of the scientific community for the possibility of: i) Biosensing of molecules using microorganisms or enzymes; ii) degrading pollutants and organic molecules; iii) producing and storing energy using microbial or enzymatic electrochemical systems (including microbial fuel cells, enzymatic fuel cells, biosupercapacitors and bio-batteries); iii) performing electrosynthesis and transformation of waste into valuable products. In our lab, joint with Prof. Franzetti @DISAT, we synthesize carbonaceous materials (biochar) starting from waste biomass and plastics and we functionalize it as anode materials or as cathode PGM-free electrocatalysts for the oxygen reduction reaction (ORR) and the hydrogen evolution reaction (HER). The main application is microbial electrochemical technology for power production, hydrogen evolution, organic removal and soil remediation. The oxygen reduction reaction (ORR) and the hydrogen evolution reaction (HER) occurring on the electrocatalysts based on carbonaceous, platinum-group metal and platinum-group-metal-free materials are evaluated.
Research Lab
Prof. Carlo Santoro
Dott. Mohsin Muhyuddin
Facilities
- Powerful and durable planetary ball mills for sample preparation of solids. Ball Milling E-max
- Rotating Ring Disk Electrode and Bipotentiostat Pine for the electrochemical characterization of electrocatalysts
- Horizontal split Furnace for performing pyrolysis processes at controlled temperature and atmosphere