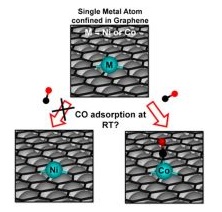
Single metal atoms confined in graphene have proven to be outstanding catalysts for various chemical reactions and promising candidates for gas sensing applications. However, it remains unclear whether their chemical activity is determined by the specific type of metal atom or is a direct consequence of the confinement itself.
In this context, a combined computational and experimental study conducted by the NanoQlab research group, led by Prof. Cristiana Di Valentin (Department of Materials Science, University of Milano-Bicocca), the Istituto Officina dei Materiali of the National Research Council in Trieste (CNR-IOM), the University of Trieste, and the Swiss Federal Institute of Technology in Lausanne (EPFL), investigated the chemical activity of metal atoms confined in graphene supported on Ni(111), using carbon monoxide as a probe molecule. At room temperature, CO stably adsorbs on Co but not on Ni. The study introduced electronic descriptors to explain this behavior, providing valuable insights for the design of graphene-based single-atom systems.
The results of this research were published in the article "CO Adsorption on a Single‐Atom Catalyst Stably Embedded in Graphene", featured in Angewandte Chemie International Edition (Impact Factor 16.1, 2023 Journal Impact Factor, Journal Citation Reports, Clarivate Analytics, 2024).
The NanoQlab research group contributed to the study through ab-initio calculations.