Research Highlights 2022
Interfacing single-atom catalysis with continuous-flow organic electrosynthesis
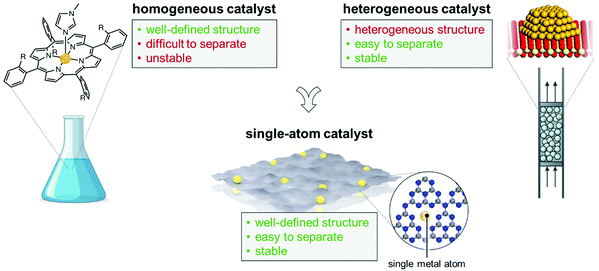
Single Atom Catalysis is a relative new frontier it the field, bridging the two worlds of homogeneous and heterogenous catalysts. A Single Atom Catalyst (SAC) is a transition metal atom atomically dispersed on a solid support. SACs are a potential platform to do a step further toward the reduction of critical raw materials and to the improvement of the catalytic activity of several key reaction processes, including organic electrosynthesis. In this review we discuss some of the progress in the field of SACs for continuous-flow organic electrosynthesis and the highlight the prospects for future applications.
Bajada, M.A.; Sanjosé-Orduna, J.; Di Liberto, G; Tosoni, S.; Pacchioni, G.; Noël, T.; Vilé, G. Interfacing single-atom catalysis with continuous-flow organic electrosynthesis. CHEMICAL SOCIETY REVIEWS 51, 3828 (2022).
Nitrate-mediated four-electron oxygen reduction on metal oxides for lithium-oxygen batteries
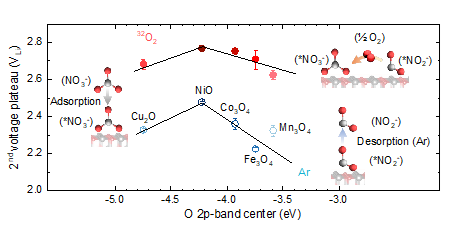
Li-O2 batteries are a promising alternative to Li-ion batteries due to the greater gravimetric energy, which is limited by poor efficiency and cycle life due to the instability of aprotic liquid electrolytes. Molten-salt electrolytes exhibit better cycling stability, higher round-trip efficiency, and form Li2O instead of Li2O2, leading to higher gravimetric energy density. In this work, in collaboration with the Electrochemical Energy Laboratory at MIT, we have shown that the apparent four-electron oxygen reduction to form Li2O in Li-O2 batteries with molten nitrate is facilitated by the electrochemical reduction of nitrate to nitrite, and subsequent chemical oxidation of nitrite to nitrate by molecular oxygen. By studying a series of transition metal catalysts by experiments and first principles calculations, we have demonstrated that optimizing the binding of nitrate to the catalyst surface enhances the kinetics of the process, with NiO-based catalyst displaying the highest discharge voltages.
Zhu, Y. G.; Leverick, G.; Giordano, L.; Feng, S.; Zhang, Y.; Yu, Y.; Tatara, R.; Lunger, J. R.; Shao-Horn, Y. Nitrate-mediated four-electron oxygen reduction on metal oxides for lithium-oxygen batteries. JOULE 6, 1887 (2022).
Oxygen reduction reaction electrocatalysis in neutral media for bioelectrochemical systems
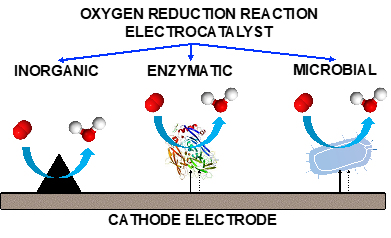
The oxygen reduction reaction (ORR) is one of the most studied reactions in the field of electrochemistry. This reaction is less studied in neutral pHs when combined with biological matter and used within bioelectrochemical systems. ORR can occur over inorganic electrocatalysts such as single atom transition metal (TM-N-C), platinum nanoparticles supported on carbon and high surface area carbonaceous materials. ORR can also occur over biotic electrocatalysts such as enzymes and microbes. The addition of biological matter increases the level of complexity of an already complex system. Each class of abiotic and biotic electrocatalysts is described highlighting the bottlenecks and limitation and identifying the limiting steps in the process. Interestingly, each class of electrocatalyst has a diverse limiting step related to the adsorption of the oxygen on the active site, the reduction of the intermediate or the rejection of the final product. Importantly, oxygen availability in gaseous form is crucial for the reaction to be efficient. Interestingly, the dimensions of these electrocatalysts are very variable spacing from angstroms (in the case of single atom) up to micrometers (in the case of microbes). Only understanding the limitation of these electrocatalysts during operation can guide the selection of the materials for a specific application.
Santoro, C., Bollella, P., Erable, B., Atanassov, P., Pant, D. Oxygen reduction reaction electrocatalysis in neutral media for bioelectrochemical systems. NATURE CATALYSIS, 5(6), 473 (2022).