Materials for electrochemical energy conversion: synthesis, ex-situ and operando characterization
Membranes and membrane-to-electrode assemblies (meas) for polymer fuel cells
Polymer fuel cells operating al low temperature (< 100°C) are the systems-of-choice for energy conversion for automotive (buses, trucks, shuttles) and for grid applications. At present, the state of the art is represented by proton-conducting devices operating with Nafion™ membranes. These fuel cells suffer of several problems, e.g. need of precious metal catalysts (e.g. platinum and platinum-group metals (PGM)), catalyst poisoning by CO at low temperature, membrane high cost. Alternative routes are offered by proton-conducting devices operating in the range 100-200°C, which based on membranes made by polybenzimidazole and related composite materials. This allows to reduce the membrane cost. Another intriguing possibility is to move towards anion (OH) conducting membranes, which allow substituting the PGM catalysts with other based on cheap elements (e.g. Fe). The research line aims at developing and characterizing both proton- and OH-conducting materials.
Solid polymer and composite electrolytes for energy storage
At present, the market of electrochemical energy storage is dominated by lithium-ion rechargeable batteries. These batteries, however, have not enough energy density, and are not really safe because of the high volatility and flammability of the organic liquid electrolyte. The quest for higher energy density can be solved by substituting the graphite anode with a lithium metal one, originating the so-called lithium metal batteries (LMB). These batteries require a solid electrolyte able to block the formation of lithium dendrites which can cause short circuits and battery faults. The availability of solid electrolytes will also help to solve the present safety problems. This research line aims at developing solid electrolytes based on functional polymers, e.g. poly(ethylene oxide) (PEO), or on polymer-ceramic nanoarchitectures.
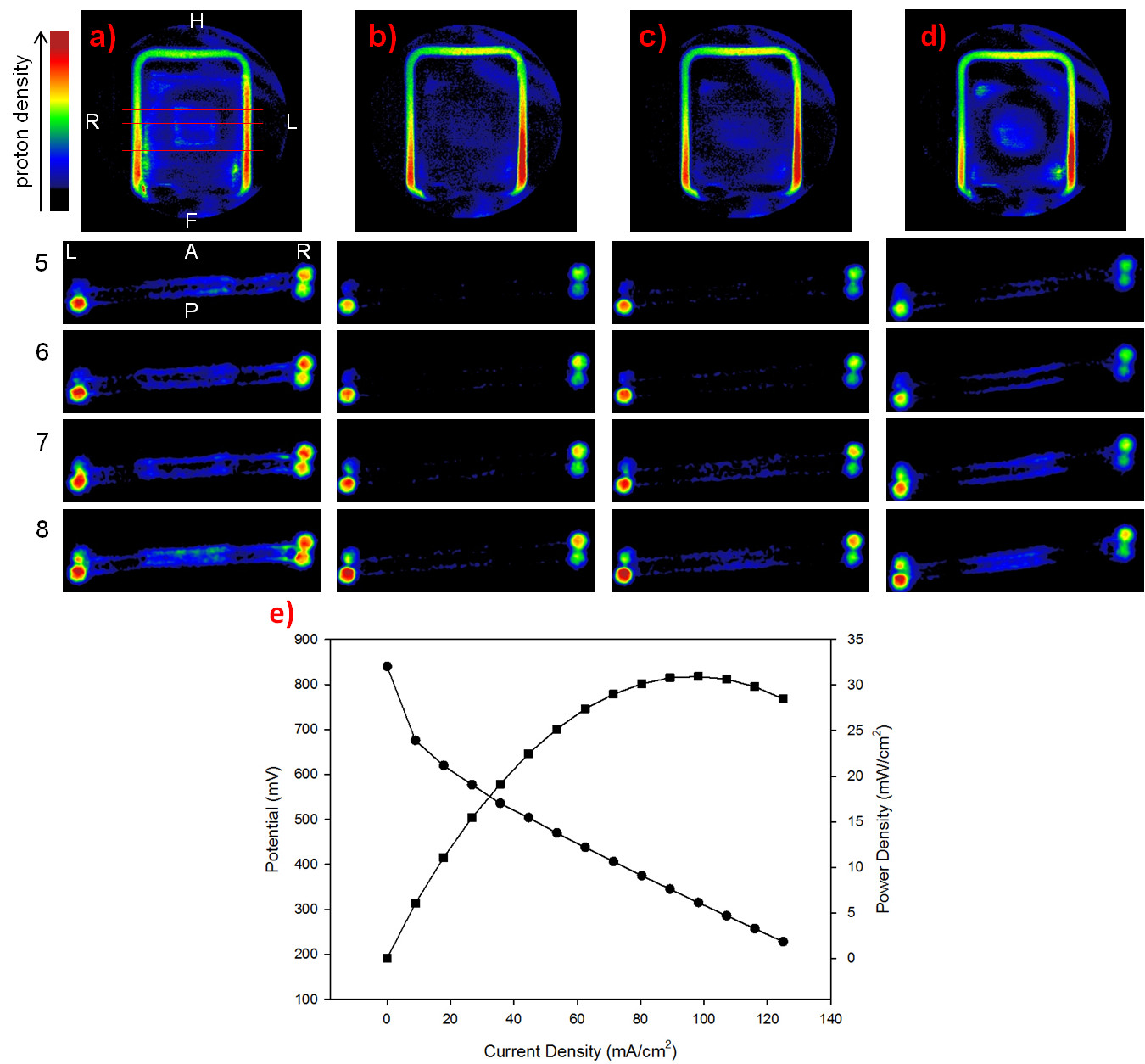
NMR/MRI operando characterization
The functional characterization of materials involved in electrochemical interfaces, or even in complete devices, cannot prescind from their study under conditions as near as possible to real operation (operando conditions). This indeed requires the use of non-destructive characterization techniques, e.g. X-rays, electron microscopies, of spectroscopies like RAMAN or NMR. This research line aims at developing and applying advanced methodologies of NMR spectroscopy and micro-imaging (MRI) to the operando investigation of materials for batteries, supercapacitors and fuel cells.
Research Group
Prof. Piercarlo Mustarelli
Dr. Chiara Ferrara