Theory of 2D and 0D materials: bidimensional layers and nanoparticles
NanoQlab is a quantum chemical laboratory focused on the investigation of nanosized and nanostructured systems for health, energy and environment by means of electronic structure calculations and molecular dynamics simulations.
Computational nanomedicine

Emerging semiconducting metal oxide nanostructures (nanospheres, nanotubes, thin films) with photocatalytic or magnetic properties are currently opening totally new horizons in nanomedicine (e.g. novel photodynamic therapies, a new class of contrast agents, magnetically guided drug delivery). We investigate shape and size dependent properties, we screen potentially efficient linkers for anchoring surfaces and binding biomolecules. We tether various kinds of biomolecules (from oligopeptides and oligonucleotides to small drugs) to the activated surface according to the desired functionality. The assembled bioinorganic systems may also be labeled with fluorescent markers and contrast agents.
Computational electrochemistry and fuel cells
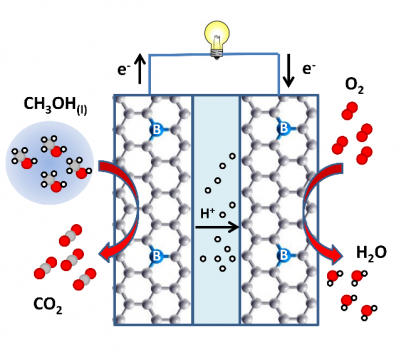
We use electronic structure calculations to design novel electrode materials for electrochemical devices and fuel cells, which are as efficient as or even more capable than precious and environmental unfriendly metal electrodes. Gibbs free energies of reaction in an aqueous environment for the all the steps of reduction (at the cathode) or of oxidation (at the anode) are computed, for example, for the oxygen reduction reaction (ORR) or for methanol oxidation reaction (MOR), respectively. Details of the reaction mechanisms and accurate cell onset- or over-potentials can be derived from the Gibbs free energy diagrams. The latter are computational quantities that can be directly compared to experimentally obtained cell overpotentials.
Catalysis under cover (2D layers)
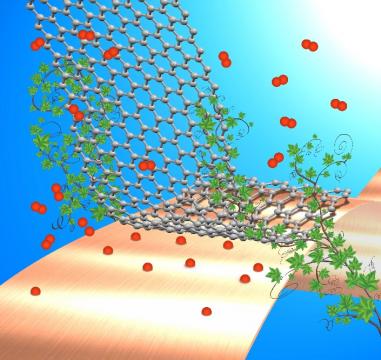
The catalysis "under cover" is a recent and emerging field of research (see Review Article by X. Bao and co. in Nature Nanotech. 2016, 11, 218), focusing the attention on the chemical reactivity taking place in the confined zone between two interfacing materials. Typically, at least one of materials is 2D, e.g. graphene, h-BN or MoS2. A number of examples of enhanced reactivity have now been reported in the literature, where the chemical process is favored if taking place between the two exposed surfaces. Still very little is known on the mechanism of this special type of catalysis and on the true role played by the two surfaces. Is the space confinement effect a sufficient reason for the enhanced reaction rate or are surface atoms actually involved in the reaction steps? Are defects and impurities also active in the promotion of chemical reactions?
Graphenic nanostructures from molecular precursors
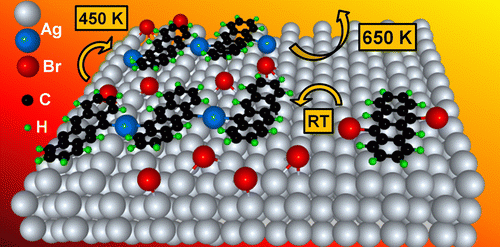
Combining density functional theory calculations with scanning tunneling microscopy and X-ray spectroscopic techniques (from our experimental partners) we investigate novel approaches for surface-assisted preparation of graphene-based nanostructures (nanoribbons, nanobowls, etc) by means of Ullmann coupling polymerization and dehydrogenation reactions of polyaromatic molecules.
Research Team
Prof. Cristiana Di Valentin
Dr. Daniele Perilli
Dr. Paulo Siani
