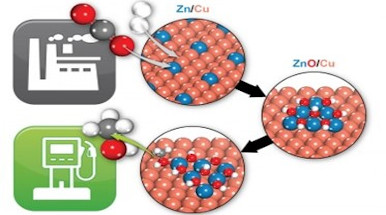
The conversion of CO2 into solar fuels (e-fuels) or other useful chemicals is at the heart of the energy transition. One of the most important processes in this sense is the production of methanol, CH3OH. For more than half a century, methanol has been produced in the world thanks to a heterogeneous catalyst, Cu/ZnO/Al2O3, where Cu nanoparticles are dispersed on an alumina support with ZnO particles as promoters. The reaction begins with syngas, a mixture of CO, CO2 and hydrogen, and the hydrogenation of carbon dioxide is an essential part of the process. Despite the technological importance and the high number of studies dedicated to the identification of the catalyst structure, several open questions still remain on the nature of the active sites and phases.
In the perspective article “From CO2 to Methanol on Cu/ZnO/Al2O3 Industrial Catalyst. What Do We Know about the Active Phase and the Reaction Mechanism?” (doi: 10.1021/acscatal.3c05669) published on ACS Catalysis (Impact factor 12.9 - 2022 Journal Impact Factor, Journal Citation Reports (Clarivate Analytics, 2023), Gianfranco Pacchioni, full professor of the Department of Materials Science of the University of Milan- Bicocca, proposes an analysis of the most accredited hypotheses on the nature of the active sites and phases of this catalyst. Among the mechanisms proposed are the formation of CuZn alloys, the migration of ZnO over the Cu nanoparticles which are covered by an ultra-thin oxide layer, the role of the Cu-ZnO interface and in particular the presence of Cu+ ions as promoters; others recognize the role of alumina as a source of acidic sites where the intermediates are stabilized or that Al ions act as dopants of the ZnO phase. The reality is that all of these mechanisms likely contribute, but under different conditions of temperature, pressure, and reactant composition. This results in a dynamic catalyst that makes the unambiguous identification of active sites difficult, if not impossible. Less controversial is the reaction mechanism, although even in this case the path followed by the reaction is a delicate function of several conditions, including the composition of the reaction mixture and the presence of water or other contaminants.