The lithium batteries we use today—so-called third-generation batteries—still rely on graphite-based anodes. While effective, graphite limits how quickly batteries can be recharged, posing one of the main obstacles to the wider adoption of electric vehicles. High charging currents can indeed cause lithium metal to deposit on the anode surface, reducing battery life and potentially creating safety risks. Finding alternative materials that can match graphite’s performance but allow for safer, faster charging is therefore a key challenge in battery research.
A research team led by Prof. Riccardo Ruffo of the Department of Materials Science, in collaboration with Ricerca sul Sistema Energetico, has developed an innovative method to prepare new anode materials for lithium and sodium batteries. The approach involves controlled oxidation of layered mixed carbides made from titanium, tin, and aluminum, producing nanostructured composites with excellent electrochemical properties.
The team’s latest results have just been published in Advanced Science (Wiley, Impact Factor 14.1, Clarivate 2024) in the article "Tailoring Oxide/MAX Phase Nanocomposites via Low‐Temperature Oxidation for Lithium‐Ion Battery Anodes: Peeking Behind the Electrochemical Mechanism via In Situ Investigations" (doi.org/10.1002/advs.202512947).
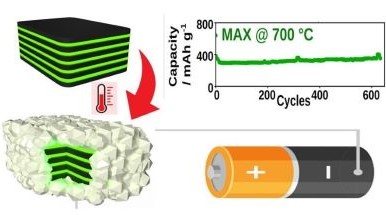
The study shows that materials obtained from controlled thermal oxidation of Ti₃Sn₀.₇Al₀.₃C₂—composed of Sn/TiO₂ nanoparticles supported on a conductive carbide matrix—can be charged and discharged within minutes, achieving performance comparable to graphite but with improved safety.