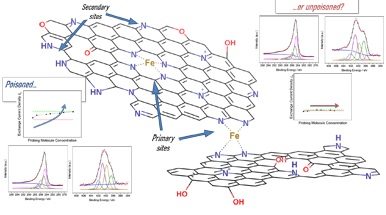
In the field of energy conversion devices operating under hydrogen and oxygen like fuel cells, the oxygen reduction reaction (ORR) is of primary importance for the future energy transition. However, ORR necessarily requires an electrocatalyst to be exploited. At the state of the art, platinum group metal-free (PGM-free) electrocatalysts based on carbonaceous nanostructures, heteroatoms, and coordinated transition metals are the most promising alternative to carbon-supported platinum nanoparticles (Pt/C), which are more expensive and more performing, but highly prone to deactivation in contaminated working environments. The comparison of the two materials is at the level of fine-tuning requiring specific activity descriptors, namely turnover frequency and site density, to understand how to improve the performance of PGM-free over Pt/C. Regarding the site density, specific probing molecules that bind with the active sites are required to evaluate the number of active sites of PGM-free electrocatalysts. However, PGM-free sites do not possess a single type of active site like in the case of Pt/C, but rather a multitude of primary (metal-containing) and secondary (metal-free) sites arising from the pyrolysis synthesis process, eventually complicating site density evaluation.
By bridging electrochemistry with X-ray photoelectron spectroscopy (XPS), establishing a relationship between performance and surface chemistry is possible. This approach has been adopted by the Department of Chemical Science and Technologies at University of Rome Tor Vergata (Dr. Valerio Ficca, Prof. Barbara Mecheri) jointly with the Department of Materials Science at the University of Milano-Bicocca (Dr. Carlo Santoro), Department of Physics at University of Rome Tor Vergata (Prof. Fabrizio Arciprete), Department of Physics, at the Sapienza University of Rome (Prof. Ernesto Placidi), Electrification and Energy Infrastructures Division, Oak Ridge National Laboratory (Dr. Alexey Serov), Department of Chemical and Biomolecular Engineering, National Fuel Cell Research Center, University of California Irvine (Prof. Plamen Atanassov). The results identified a method for evaluating the direct interaction (through chemisorption) of probing molecules over the PGM-free primary and secondary sites. The discrimination of such interactions is of paramount importance in an effective evaluation of site density, leading to an improved understanding of the nature of active sites. In addition, upon identification of the interaction phenomenon, it will be possible to choose contaminants capable of selective interaction with the active sites of interest, boosting site density evaluation.
The results of the study are reported in the paper "Exchange Current Density as an Effective Descriptor of Poisoning of Active Sites in Platinum Group Metal-free Electrocatalysts for Oxygen Reduction Reaction" (DOI: 10.1021/acscatal.2c05222), published on ACS Catalysis (Impact Factor 13.700, Journal Citation Report (Clarivate Analytics, 2021)).