Abiotic and biotic electrocatalysts are crucial for the reduction of oxygen in neutral media associated to bioelectrochemical systems. This interdisciplinary field of study covers electrocatalysis, electrochemistry, inorganic electrocatalysis, bioelectrochemistry, engineering of electrodes and transport phenomena at different spatial scale. An international team of experts summarized the main results in this wide field in the review “Oxygen reduction reaction electrocatalysis in neutral media for bioelectrochemical systems” (DOI: s41929-022-00787-2) published in Nature Catalysis (Impact Factor 40.706, Journal Citation Reports (Clarivate Analytics, 2020)), identifying the bottleneck and drawing the roadmap for practical application in bioelectrochemical systems. This review was led by Dr. Carlo Santoro, head of the Electrocatalysis and Bioelectrocatalysis Laboratory (EBLab https://ebl.mater.unimib.it/) of the Department of Materials Science at the University of Milano Bicocca jointly with Dr. Paolo Bollella (University of Bari Aldo Moro), Dr. Benjamin Erable (Laboratoire de Genie Chimique CNRS Toulouse), Prof. Plamen Atanassov (University of California Irvine) and Dr. Deepak Pant (Flemish Institute for Technological Research (VITO)).
Why oxygen reduction reaction is important?
The oxygen reduction reaction is one of the most studied reactions in the field of electrochemistry mainly related to fuel cells, batteries, sensing and corrosion. Oxygen as reagent is very precious because is readily available, at practically no cost and weight since it is present abundantly and naturally in the atmosphere and it has a high red-ox potential which is beneficial for high voltage output. However, this reaction is less studied in neutral pHs when combined with biological matter such as enzymes and bacteria and used within bioelectrochemical systems.
What is the advantage of this reaction coupled in bioelectrochemical systems?
Bioelectrochemical systems are used for treating wastewater, transform organics, producing valuable added products, desalination and sensing. They rely on biotic matters at the anode electrode composed by enzymes or bacteria capable of using organic matter as fuel and simultaneously degrading organic molecules and producing a flow of electrons (e.g. electricity). The circuit is then closed with the reduction of oxygen occurring at the cathode. Oxygen reduction reaction is then crucial for these systems to operate. Improving the oxygen reduction reaction, that often is the limiting step, would also improve the efficiency of the overall bioelectrochemical system. Few examples of electrocatalysts integrated into large scale electrodes are reported and one of them is from the Flemish Institute for Technological Research (VITO), where meter squared of electrodes are fabricated.
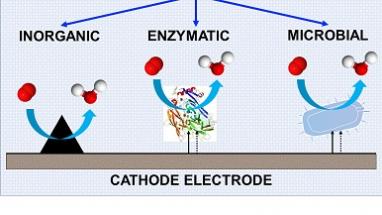
What are the main electrocatalysts used for the reduction of oxygen?
In this review, we combined diverse know-how and experience matured in the past 10-15 years related to the oxygen reduction reaction occurring over inorganic electrocatalysts such as single atom transition metal electrocatalysts, platinum nanoparticles supported on carbon, high surface area carbonaceous materials. Also, biotic electrocatalysts such as enzymes and microbes are explored. The addition of biological matter increases the level of complexity of an already complex system. We have described each class of abiotic and biotic electrocatalysts highlighting the bottlenecks and limitation and identifying the limiting steps in the process. Interestingly, each class of electrocatalyst has a diverse limiting step related to the adsorption of the oxygen molecule on the active site, the reduction of the intermediate or the rejection of the final product. Importantly, the availability of oxygen in gaseous form rather than in dissolved form is crucial for the reaction to be efficient. It must be underlined that the dimensions of these electrocatalysts are very variable spacing from angstroms (in the case of single atom) up to micrometers (in the case of microbes).
Which electrocatalyst is the most performing in neutral pH electrolyte?
Despite all these electrocatalysts have their own advantages and disadvantages, create a well-defined and fair ranking on these materials is quite complex. Enzymatic electrocatalysts have lower losses compared to the other materials but cannot produce high current. Moreover, they are quite expensive and exhibit a short life-time. Among the inorganic electrocatalysts, the best compromise between cost, performance and durability is given by single atom transition metal electrocatalysts which they look resilient to be poisoned by anions. Bacterial electrocatalysts are the most complex electrocatalyst and probably the less studied and understood. However, also these electrocatalysts seems promising for long terms operations as they are resilient. Only understanding the limitation of these electrocatalysts during operation can guide the selection of the materials for a specific application.