The material breathes in and out on command by means of a switch. Interestingly, the porous framework breathes by capturing and releasing carbon dioxide only when it receives a luminous stimulus at a specific wavelength. It is designed thanks to the collaboration of the research teams led by the Nobel Prize winner Ben Feringa, and by Prof. Sander Wezenberg of the University of Groningen and by Prof. Angiolina Comotti, full professor of Industrial Chemistry of the Department of Materials Science of the University of Milan – Bicocca. The research results “Modulation of porosity in a solid material enabled by bulk photoisomerization of an overcrowded alkene” (doi:10.1038/s41557-020-0493-5) have been recently published in the journal Nature Chemistry .
Common materials have multiple properties and functions which generally can vary with temperature or pressure. The real challenge for researchers is to design materials that are able to respond to a stimulus and perform an action by inserting a switch on the nanometer scale sensitive to a specific command. The molecular switch is a molecule that can receives a stimulus or a command and activates a function.
Inspired by biological systems capable of developing dynamic processes, in recent years numerous research groups have developed several machines and artificial molecular switches in solution capable of performing certain functions. The isotropic thermal motion, however, precludes any form of collective action and macroscopic work. On the contrary, the organization of solid-state machines and molecular switches are able to transform nanoscopic changes stimulated by light or heat into work of practical utility. Therefore, the current challenge is to organize machines and molecular switches that can perform selected and non-randomized rotary or switching motions.
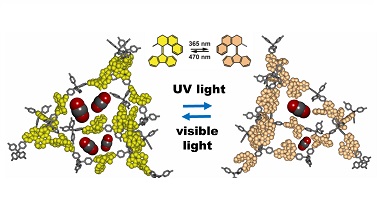
The challenge was collected and won by the research groups of the University of Groningen and the University of Milan - Bicocca who have exploited materials with high porosity thanks to which they can incorporate molecular switches, providing the free volume essential for dynamic motions. The material behaves as an armor for flexible molecular components: the light and porous scaffold does not prevent the motion of the molecular switch which is controlled by light / heat. CO2 capture is therefore modulated by light and / or heat due to the quantitative photoisomerization of the molecular switch.
These new prototype materials lead to the generation of macroscopic properties such as the regulation of the quantity of absorbed CO2 controlled by external stimuli. This discovery also opens up new opportunities in the field of chiral molecule separation technologies in the presence of a molecular switch that modulates the pore size.
The importance of this research is also highlighted by the journal Nature Chemistry itself by dedicating a section of the News & Views section entitled "Photoswitching to the core".
Why is the carbon dioxide capture on command so important?
The absorption of carbon dioxide which is very harmful to the human environment and unfortunately generated by industrial and transport activities is very topical - explains Angiolina Comotti. - The problem is that CO2 must not only be captured, but must be released on command to be reused and / or transformed into products with higher added value. The process of releasing CO2 is important, otherwise the absorbent materials would saturate and would not work further.
The material designed thanks to this research 'breathe' on command, that is, it inhales absorbing CO2 and exhales releasing it through light stimuli at specific wavelengths. In this way the material swells with CO2 immediately after receiving the specific order and then "squeezes" to a second specific stimulus, such as the lungs behind the pressure of the chest.
How did you design and make the porous material so that it also included the molecular switch?
We have given priority to the stability of the whole framework which guarantees many breathing cycles. So we preferred the most stable bonds to temperature and humidity (the covalent bonds): to use these we had to use effective reactions that formed a porous and swelling three-dimensional network like a sponge capable of absorbing CO2. However, this network formation reaction must also include the element that obeys orders and transmits the modification induced to the entire material, the actual switch, which is naturally a delicate "organ". This chemical process of assembling the three-dimensional network, however, does not destroy the active molecular element.
The project supports a challenge currently very active in the field of materials chemistry, that of producing solids with active properties or modulated on command, so that a stimulus produces a useful behavior.
For the switch to be efficient, the reversibility of the transformation is a crucial factor. What result did you get?
The transformation of the switch in the two states obtained by irradiation at the appropriate ultraviolet frequencies is 100% reversible - explains Prof. Angiolina Comotti. - We obtained the relevant CO2 capture values from the CO2 absorption measurement with a suitable apparatus (for absorption isotherms). We have verified that the three-dimensional framework is rearranged by the state of the switch and modulates the amount of CO2 absorbed.
An important and uncommon aspect is represented by the fact that the process is massive and takes place in a solid - concludes Prof. Silvia Bracco. - Nuclear magnetic resonance, which is typically performed on patients, allowed to follow the process in the solid and demonstrated the quantitative transformation (photoisomerization in the specific case) of the switch.