Lithium batteries have changed and are changing our society: almost all the portable devices we know have been developed thanks to these power sources. However, this technology is destined for an increasingly limited use in the future due to the high costs and the scarce availability of some raw materials (such as lithium itself). A possible alternative is the development of rechargeable sodium batteries, a more abundant element in nature. One of the most severe issue that researchers are facing is to find the proper negative material because graphite (the most common anode in lithium batteries) exfoliates when reduced in presence of Na+.
A group of researchers from the Department of Materials Science of the University of Milan - Bicocca led by Prof. Riccardo Ruffo, associate professor of Physical Chemistry, thanks to the collaboration with Ricerca Sistema Energetico (RSE) and with the University of Ulsan (South Korea), has developed MXene-based compounds which meet the requirements as "negative materials" of the future in rechargeable sodium batteries. In the paper "Enhanced Functional Properties of Ti3C2Tx MXenes as Negative Electrodes in Sodium ‐ Ion Batteries by Chemical Tuning" (doi: 10.1002/smtd.202000314) published in the journal Small Methods (Impact factor 12.190, - 2019 Journal Impact Factor, Journal Citation Reports - Clarivate Analytics, 2020), researchers have shown how these materials have lifetimes, efficiencies, and power densities that are the state of the art for this application.
What are MXene and where they can be applied?
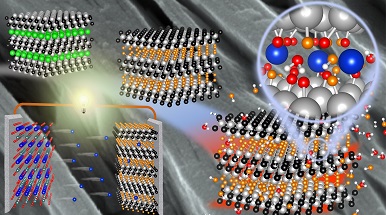
MXenes are lamellar structures with large spaces between the layers (1 nm) which makes them bi-dimensional materials with a wide spectrum of application - explains Riccardo Ruffo - and suitable for intercalation of ions. Just think that in graphite, a material widely used as an intercalation electrode, the space between the layers is about 0.3 nm. In the term MXene, M stands for the transition metal (Ti, V, Cr, Mo), X for carbon (C) or nitrogen (N), while the suffix -ene is reminiscent of the graphene structure. In practice, they can be defined as transition metal carbides or nitrides with a graphene structure. Among them, we investigated the titanium carbide of chemical formula Ti3C2Tx where T stands for the terminal groups of the lamellae (-F, -OH, etc.).
MXenes have excellent electronic conductivity, a hydrophilic surface and good mechanical properties, and they have been studied as transparent electronic conductors, membranes for ion separation, hydrogen storage materials, catalysts for the evolution of hydrogen and above all electrodes in energy storage , both in capacitors and in alkaline ion rechargeable batteries (lithium and sodium batteries).
Which methodology did you use?
The approach was highly multidisciplinary. The work is the frame of the project "Departments of Excellence: Materials for energy" and this has promoted collaboration between researchers from the department with different backgrounds.
The work is the result of years of research, which covers the entire value chain: from the preparation of the MAX precursor (Ti3C2Al) to the synthesis of MXene (Ti3C2Tx), to its chemical, structural and morphological characterization, up to the formulation in anodes for sodium ion rechargeable batteries, to get the functional properties in battery prototypes. To better understand the correlations between the structure and the electrochemical properties, the electronic features were also studied with ab-initio modelling.
How important were the extra-departmental collaborations?
The collaboration with RSE was fundamental, because it allowed us to get in touch with these fascinating materials and to have the quantities of MAX precursor to tune the MXenes preparation to improve their properties. The collaboration with Korean colleagues was equally important because it gave us access to surface characterization techniques (XPS) which are not available in the department and which are fundamental in this area.