The conversion of solar light into chemical energy is at the basis of photocatalysis, aiming at transforming low-value molecules into useful products. How to improve the photocatalysis efficiency and promote the migration of energy carriers toward active centres? A recent computational study by a group of researchers from the Department of Materials Science of the University of Milano – Bicocca, led by Prof. Gianfranco Pacchioni, showed that the atomistic structure and the nature of the interface of a composite material plays a key role, explaining previous theoretical data hardly reconciling with experimental evidences.
The results of this research have been recently published on Advanced Functional Materials (Impact factor 16.837 - 2019 Journal Impact Factor, Journal Citation Reports (Clarivate Analytics, 2019)) in a communication titled "Nature and Role of Surface Junctions in BiOIO3 Photocatalysts” (doi:10.1002/adfm.202009472).
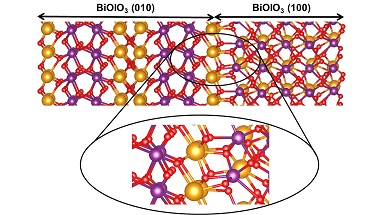
In their study, the members of the Quantum Chemistry Lab (QCLab) led by Prof Pacchioni, designed a computational study of the interfaces composed by BiOIO3, a recently discovered photocatalyst. They showed that the surface termination at the interface plays a primary tole on the efficiency of the system, an aspect hardly to be inferred without an atomistic model of the interfaces’ nature.
Composite systems, made by at least two materials, often overperform their components, due to the promotion of the charge carriers’ species toward active sites at the contact region. An atomistic description of the contact region, also called interface, is a challenging purpose, given its confinement in the inner part of a material. In silico experiments (simulations) may often help to predict the atomistic structure and chemical nature of an interface and help into rationally designing ones able to efficiently separate charge carriers.
How can a material convert solar energy in a form suitable for human needs?
Solar energy is the main renewable source – tells us Giovanni Di Liberto, a researcher of the Department of Materials Science and QCLab member. – Photons (quanta of radiation) may be converted into various other forms of energy (thermal, electrical, chemical), in presence of a device capable to capture them; this process is at the basis of, for instance, clorophyll photosynthesis occurring in plants. One of the hot topics in materials science is the study of new devices able to mimic this process. We can devide it into three main steps: i) photons’ capture, ii) energy transport through charge carriers’ separation, iii) solar light conversion. When a material absorbs the radiation, some electrons hosted into atomic orbitals are promoted into higher energetic levels, otherwise impossible to populate. At the same time, this excitation implies the generation of a second species, named holes, having an opposite charge. Electrons and holes diffuse through the material carrying around the absorbed energy. If charge carriers migrate toward active sites, the energy can be converted into, for instance, electrical energy (solar cells) or chemical one (photocatalysis). However, this process is hindered by the further reaction, called recombination, where electrons and holes recombine and energy is dispersed. Here the interface may play a role.
How an interface works in composite materials?
When two materials are joint, they can create an interface, or junction, – explains Sergio Tosoni, a researcher of the Department od Materials Science and QCLab member. – A channel can open between their electronic levels. If the energy levels of one components are capable to stabilize electrons, and those of the second may stabilize holes, then electrons-holes separation becomes energetically favourable. This event decreases the recombination probability, thus favouring the migration process towards active sites. Thanks to the rational design of the interfaces it is possible to tailor the separation in an efficient way, and also to control its direction, i.e. determining towards which components electrons and holes migrate.
What is the contribution of computational chemistry in this field?
The description of an interface at the atomistic level is a challenging purpose, – shows Giovanni Di Liberto –. Quantum chemistry simulations allows to investigate events in this scale, providing results in time span comparable to those of an experiment.
Furthermore, simulations allow to study different interfaces, their structure, stability, and electronic properties, prior to dedicate a lot of work in synthesizing them. For instance, it is possible to predict that the structure and the properties at the interface will differ from those of its separated components, with strong effects on the behaviour of the composite.