Protein misfolding and aggregation are the fundamental causes of many degenerative conditions, including Alzheimer’s and Parkinson’s disease (AD and PD) as well as type II diabetes. Misfolded proteins are usually biologically inactive; however, the dynamic equilibrium between monomeric and oligomeric aggregates may lead to cytotoxic states that induce the degenerative condition. In AD, amyloid-β (Aβ) and tau proteins misfold and form oligomers and fibrils that accumulate in the brain in pathogenic pathways leading to synaptic loss and selective neuronal cell death.
Rich in cross β-sheet conformations, soluble oligomers of Aβ are suspected to be more deleterious than Aβ fibrils and plaques and they appear prior to tau accumulation and the clinical symptoms. Therefore, soluble Aβ oligomers have been recently tested as potential targets for development of AD-specific drugs, with the aim to stop the decline of the patient’s cognitive functions. Translation from mouse to man has been demonstrated with antibody-based drugs, and the monoclonal antibodies Aducanumab and Lecanemab have been recently approved by the Food and Drug Administration (FDA) for early AD treatment. Controversy over efficacy and side effects, such as microhemorrhages and brain swelling, still highlight the urgent need for improved therapy and novel diagnostic tools for assessing the treatment efficacy. In search of an alternative and non-invasive therapy, singlet oxygen (SO) generated by suitable photosensitizers and visible light were demonstrated to inhibit Ab aggregation and toxicity thus achieving a peculiar photodynamic therapeutic effect. However, this strategy is largely impractical in AD treatment due to limited penetration of high energy photons through tissues and skull.
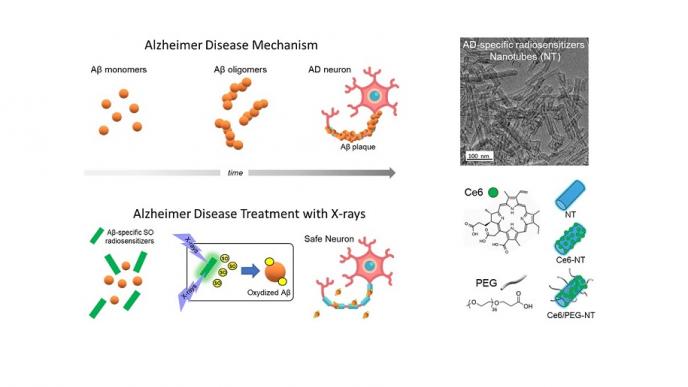
The research team of the University of Milano-Bicocca (Dr. Valeria Secchi, Dr. Francesca Cova, Dr. Irene Villa, Prof. Marcello Campione) led by Prof. Angelo Monguzzi of the Department of Materials Science in collaboration with Prof. Shai Rahimipour at the University of Bar-Ilan in Israel, developed a revised strategy to overcome this limitation by achieving SO sensitization and inhibition of Aβ oligomers aggregation using a combination of multicomponent hybrid scintillating nanotubes (NTs) and low doses of highly penetrating X-rays. The NTs are functionalized with a SO photosensitizer that preferentially target and interact with Aβ oligomers, and with polyethylene glycol (PEG) molecules to increase water solubility and to minimize unselective interaction with other biomolecules. The presence of the dense NTs enhances a localized deposition of the ionizing radiation and the activation of the photosensitizers, thus locally boosting the production of the SO in close proximity of Aβ in the deep brain. Consequently, the oxidation of Ab monomers and early oligomers prevents their aggregation into the toxic forms. In an optimized composition, the aggregation of Aβ was reduced by more than 80% employing the NTs and soft X-ray exposure. In transgenic Caenorhabditis (C.) elegans AD models in vivo, the combination of NTs and X-ray dramatically reduced the amount of Aβ oligomers and recovered the functional and behavioral symptoms associated with Aβ aggregation and toxicity. The results demonstrated here suggest that the proposed X-ray-based approach exploiting Aβ-targeting SO radiosensitizers can be a revolutionary and unexplored strategy to treat AD and inhibit its progression.
The results of the research are reported in the paper “Towards non-invasive treatment of Alzheimer’s disease using scintillating nanotubes” (DOI: 10.1002/adhm.202301527) published in Advanced Healthcare Materials (Impact Factor 10.0, Journal Citation Report 2022 (Clarivate Analytics, 2023)).