Quick and clean chemical reactions limiting the usage of toxic or hazardous reactants and solvents, enabled by innovative single-atom catalysts. A team of researchers from the Materials Chemistry and Chemical Engineering Department (Politecnico di Milano) and the Quantum Chemistry Laboratory at the Department of Materials Science (University of Milano-Bicocca) reached this goal. Dr. Giovanni Di Liberto, Prof. Sergio Tosoni and Prof. Gianfranco Pacchioni took side in this research joining experiments and theoretical modelling, permitting to shed light on the relationship between the coordination of the metal atom and its catalytic performance.
The results were recently published in ACS Catalysis, one of the best scientific journal in the field of catalysis (Impact factor 13.084 - Journal Citation Reports, Clarivate Analytics, 2020) in a paper entitled “Azide-Alkyne Click Chemistry Over a Heterogeneous Copper-Based Single-Atom Catalyst” (doi: 10.1021/acscatal.1c05610).
What do you mean with “click chemistry”?
It is a common expression in the pharmaceutical chemistry community, starting from the early 2000s - explains Giovanni Di Liberto, - and it refers to clean, quick, and highly selective chemical reactions, not requiring any further steps to purify the product or get rid of a toxic solvent: a chemical process as easy as shopping online, with “just one click”. These reactions are highly requested wherever a wide database of chemical compounds needs to be sampled, as in the case of pharmaceutical research, to screen out a large number of molecules potentially useful in the development of a new drug. In this specific case, we deal with an azide-alkine cycloaddition, forming a heterocyclic molecule. The heterocycles are, in turn, useful building blocks for more complex molecules.
What is a single-atom catalyst, and why is it relevant?
It is a single atom embedded in a solid matrix, enabled to accelerate a chemical reaction – explains Sergio Tosoni -. Traditionally, we distinguish between homogeneous catalysts (i.e., a species in the same aggregation state as the reactants) and heterogeneous catalysts (a catalytically active cluster or nanoparticle anchored on an inert support). The homogeneous catalysts are often very active, albeit not very selective. Moreover, it can be hard to recover the catalyst after the reaction, which implies a loss of metallic material that should rather be reused. The particles composing the heterogeneous catalysts, on the contrary, often display a limited activity; in particular, their activity depend on their size. Unfortunately, these particles tend to aggregate and become inert in the reaction’s environment. The single-atom catalyst, somehow, displays the advantages of both type of catalyst: on the one hand, it represents the lowest size limit of a catalytically active particle, on the other, it is stably anchored to an inert support (carbon nitride in the present case), which prevents the loss of precious material.
How did the Quantum Chemistry Laboratory and our Department contribute to this work?
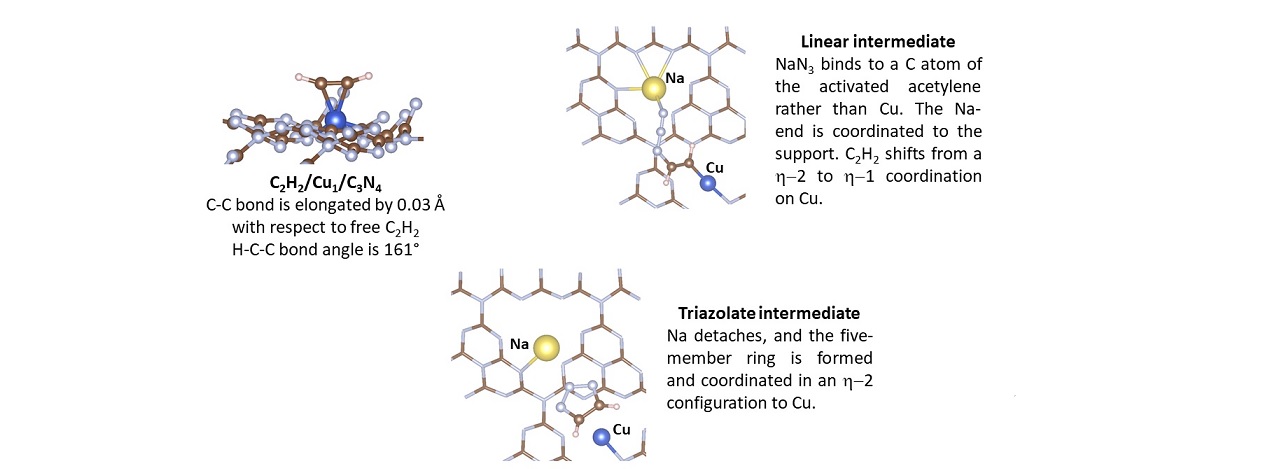
The reaction was carried out in the Laboratories at the Materials Chemistry and Chemical Engineering Department, Politecnico di Milano. We provided a structural modelling of the single-atom catalyst, and we illustrated the reaction mechanism by means of computer simulations – adds Gianfranco Pacchioni -. Copper can be stabilized on various sites in the carbon nitride matrix. Each site is characterized by a specific coordination of the Cu atom, strongly influencing its reactivity. The key-step of the chemical process is the activation of the alkine molecule: once the kinetic barrier to activate the C-C triple bond is overcome, the reaction proceeds along a favourable free-energy profile. The second reactant is coadsorbed on the Cu single atom, and then the cycloaddition takes place. It is worth remarking the added value of the synergy between experiment and simulation. If such a study were carried out exclusively by means of computer simulations, it would lead to barely speculative conclusions. Viceversa, the experimental results, in absence of any theoretical modelling, would confirm that the reaction actually took place, but could not provide a full insight on the relationship between the chemical structure and the catalytic activity, which is the base for the design of any new catalytic material.