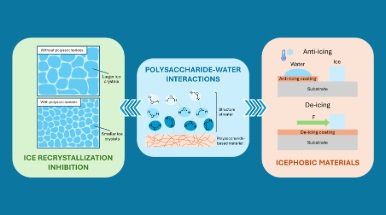
It is always time to talk about ice, even in these hot summer days. If you fancy a smooth ice cream, then you may know that its texture can be controlled by a specific class of natural materials, i.e. carbohydrates. Specifically, polysaccharides, biopolymers consisting of long chain of saccharides, commonly known as sugars, can be used to control the size of ice crystals (spoiler: you want them to be as small as possible for a smooth ice cream).
In the recent review “Water-polysaccharide interactions and their properties in freezing conditions” (DOI: 10.1016/j.carbpol.2025.124138),, published by Carbohydrate Polymers (Elsevier, Impact Factor 12.5, 2024 Journal Citation Reports (Clarivate Analytics, 2025)), the authors outline the current understanding regarding the complex interactions of polysaccharides with water, especially at subzero temperatures, a topic of interest across many fields, from materials science and engineering, to chemistry, biotechnology and food science. The review first focuses on water structure at the interface with polysaccharides and on the main characterization techniques, and then expands the discussion on the properties of polysaccharide-based materials in freezing conditions.
Federica Marelli, Prof. Carlo Antonini and Dr. Irene Tagliaro of the Department of Materials Science of the University of Milano-Bicocca, conducted the work in collaboration with Daniele Pontoriero, a Master student in the Department of Biotechnology and Biosciences. The authors will soon publish their research on a unique interaction between ice and polysaccharide this monolayers, a thin as 10 nm (less than one thousands of human hair). Stay tuned!