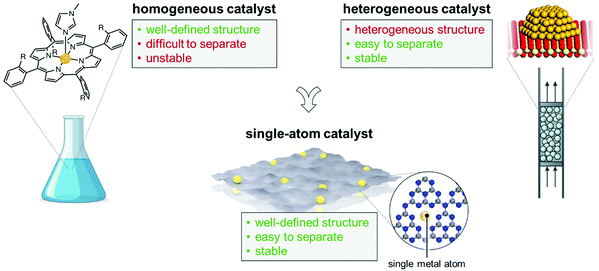
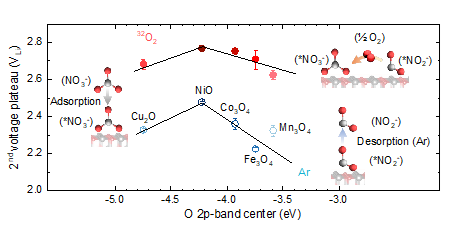
Li-O2 batteries are a promising alternative to Li-ion batteries due to the greater gravimetric energy, which is limited by poor efficiency and cycle life due to the instability of aprotic liquid electrolytes. Molten-salt electrolytes exhibit better cycling stability, higher round-trip efficiency, and form Li2O instead of Li2O2, leading to higher gravimetric energy density. In this work, in collaboration with the Electrochemical Energy Laboratory at MIT, we have shown that the apparent four-electron oxygen reduction to form Li2O in Li-O2 batteries with molten nitrate is facilitated by the electrochemical reduction of nitrate to nitrite, and subsequent chemical oxidation of nitrite to nitrate by molecular oxygen. By studying a series of transition metal catalysts by experiments and first principles calculations, we have demonstrated that optimizing the binding of nitrate to the catalyst surface enhances the kinetics of the process, with NiO-based catalyst displaying the highest discharge voltages.
Zhu, Y. G.; Leverick, G.; Giordano, L.; Feng, S.; Zhang, Y.; Yu, Y.; Tatara, R.; Lunger, J. R.; Shao-Horn, Y. Nitrate-mediated four-electron oxygen reduction on metal oxides for lithium-oxygen batteries. JOULE 6, 1887 (2022).